Axitinib (AG013736; trade name Inlyta) is a small molecule tyrosine kinase inhibitor developed by Pfizer. It inhibits multiple targets, including VEGFR-1, VEGFR-2, VEGFR-3, platelet derived growth factor receptor (PDGFR), and cKIT (CD117). It has been shown to significantly inhibit growth of breast cancer in xenograft models and has been successful in trials with renal cell carcinoma (RCC) and several other tumor types.
On January 27, 2012, the U.S. Food and Drug Administration (FDA) approved axitinib for use in patients with renal cell carcinoma that had failed to respond to a previous treatment. After that, Evershine Chemical began development and now we can provide different intermediate for research purpose.
All the product related to Axitinib is for research purpose only but not for commercial purpose.
Axitinib
AG013736, N-Methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide |
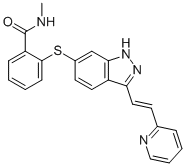 |
319460-85-0 |
Axitinib N-1 intermediate
(E)-N-methyl-2-(3-(2-(pyridin-2-yl)vinyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-6-ylthio)benzamide |
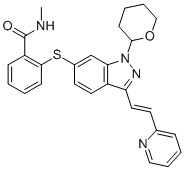 |
885126-35-2 |